Making carboxyl(ate) friends
When it comes to supramolecular chemistry, the carboxylic acid group (and its conjugate carboxylate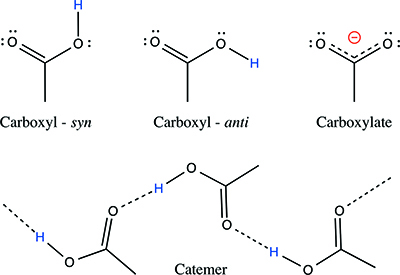 base) is one of the chemist's most flexible friends. In pairs, they act as supramolecular synthons from which more complicated structures might be built but also offer up complex hydrogen bond connectivity. Luigi D'Ascenzo and Pascal Auffinger of the University of Strasbourg, France [D'Ascenzo, L. & Auffinger, P. (2015), Acta Cryst. B71, 164-175; doi: 10.1107/S205252061500270X], point out that until now there has been no exhaustive classification of these carboxyl(ate) motifs present in crystal structures, despite their prevalence and the fact that carboxyl(ate)s are among the most well-studied hydrogen bonding groups.
base) is one of the chemist's most flexible friends. In pairs, they act as supramolecular synthons from which more complicated structures might be built but also offer up complex hydrogen bond connectivity. Luigi D'Ascenzo and Pascal Auffinger of the University of Strasbourg, France [D'Ascenzo, L. & Auffinger, P. (2015), Acta Cryst. B71, 164-175; doi: 10.1107/S205252061500270X], point out that until now there has been no exhaustive classification of these carboxyl(ate) motifs present in crystal structures, despite their prevalence and the fact that carboxyl(ate)s are among the most well-studied hydrogen bonding groups.
D'Ascenzo and Auffinger have now used what they describe as "simple stereochemical considerations" to identify just seventeen association types: thirteen carboxyl-carboxyl and four carboxyl-carboxylate motifs. This small number emerges from their analysis despite the seemingly overwhelming diversity of carboxyl–carboxyl(ate) dimers reported. To do so they took into account the free rotation that can take place around the hydrogen bond formed between the syn (C-O-H angle between 0 and 120 degrees) and anti (C-O-H angle between 120 and 180 degrees) carboxyl conformers and the syn and anti lone pairs of the oxygen atoms. They gleaned from this a simple rule that it is only possible for eight distinct catemer motifs (polymeric-like chains of carboxyl groups in the crystal) to form. They have identified examples of all dimers and catemers in compounds for which crystal data are recorded in the Cambridge Structural Database (CSD).
The researchers emphasize how the analysis of high-resolution structures of small molecules containing hydrogen atoms could offer new insights into the properties and behavior of much larger and far more complex biomolecular systems, the structures for which have been determined only at low resolution. They added that precise characterization and classification of these supramolecular motifs has implications for crystal engineering, pharmaceutical research (in particular drug co-crystallization) and the biomolecular sciences where related moieties are found, for instance, in the tertiary structures of proteins, in which hydrogen bonded pairs of amino acids or ligands containing carboxyl(ate) groups are present.
The team has not only classified the full gamut of dimers and catemers, but provided a systematic naming system, or nomenclature, for these and defined the recurrent hydrogen bonding themes among them. Despite their efforts to simplify the concept of carboxyl-carboxyl(ate) dimers and catemers that exist, they remain "astonished" that cyclic dimers do emerge rather than the single, simple hydrogen bonded dimers. Indeed, the cyclic dimer is actually the most prevalent motif.
Of course, classification, categorization and simplification do not necessarily provide a workaround for the creation of designer crystals. As crystal engineering pioneer Gautam Desiraju noted in 2007 on witnessing the constant discovery of unforeseen structures and assembly motifs, "it would seem that the brute force method will eventually win". Some rules do not always apply, some rules are there to be broken and in some circumstances these rules are just too complex to be comprehended and to guide the construction of supramolecular structures and novel crystals by chemists.


